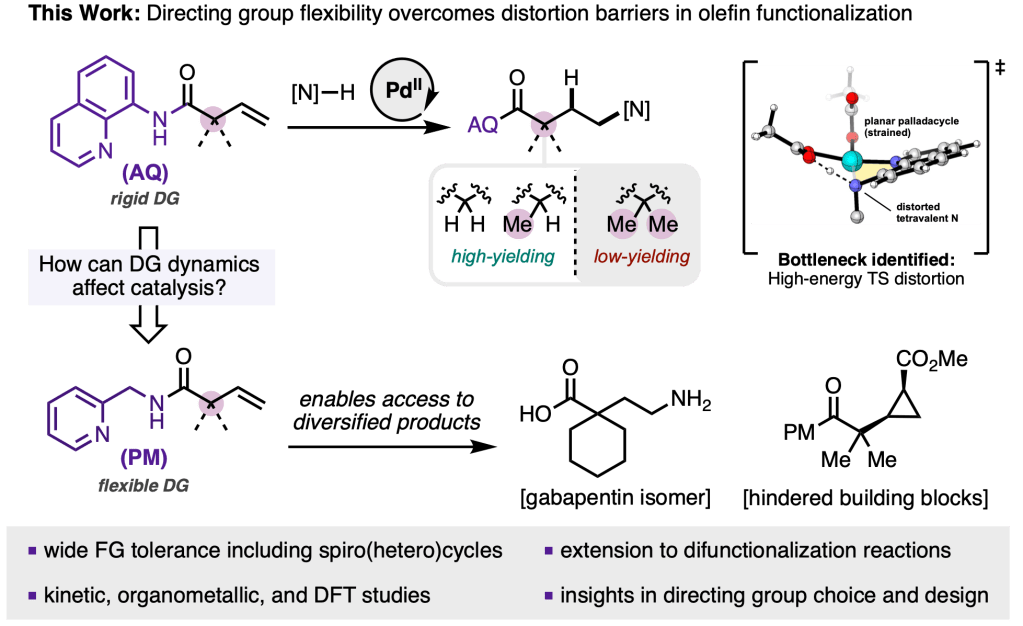
In our latest pre-print, we explore the origins of diminished reactivity of α,α-gem-disubstituted alkenyl amides through a combination of kinetics, organometallic synthesis, and density functional theory, allowing us to pinpoint the rigidity of our standard 8-aminoquinoline amide directing group as problematic in a key step in the catalytic cycle. Switching to a simple flexible directing group leads to >115x rate acceleration and dramatic expansion of substrate scope. The work was made possible through a multi-institutional team from Scripps Research (Engle and Blackmond labs), the University of Pittsburgh (Liu lab), Bristol Myers Squibb, Enamine! Congrats to the project co-leads, Al, Gift, and Shijia—as well as the entire team.
For a link to the pre-print in ChemRxiv, click here: https://chemrxiv.org/engage/chemrxiv/article-details/69368ccda10c9f5ca1daf3bf